By
Shubham Sunil Malu
ABSTRACT
Self-healing materials are a class of smart materials that have the structurally incorporated ability to repair damage caused by mechanical usage over time. The inspiration comes from biological systems, which have the ability to heal after being wounded. Initiation of cracks and other types of damage on a microscopic level has been shown to change thermal, electrical, and acoustical properties, and eventually lead to whole scale failure of the material. Usually, cracks are mended by hand, which is unsatisfactory because cracks are often hard to detect. A material (polymers, ceramics, etc.) that can intrinsically correct damage caused by normal usage could lower production costs of a number of different industrial processes through longer part lifetime, reduction of inefficiency over time caused by degradation, as well as prevent costs incurred by material failure. For a material to be defined strictly as self-healing, it is necessary that the healing process occurs without human intervention. Some examples shown below, however, include healing polymers that require intervention to initiate the healing process.
A good way to enable multiple healing events is to use living (or unterminated chain-ends) polymerization catalysts. If the walls of the capsule are created too thick, they may not fracture when the crack approaches, but if they are too thin, they may rupture prematurely.
In order for this process to happen at room temperature, and for the reactants to remain in a monomeric state within the capsule, a catalyst is also imbedded into the thermoset. The catalyst lowers the energy barrier of the reaction and allows the monomer to polymerize without the addition of heat. The capsules (often made of wax) around the monomer and the catalyst are important maintain separation until the crack facilitates the reaction.
There are many challenges in designing this type of material. First, the reactivity of the catalyst must be maintained even after it is enclosed in wax. Additionally, the monomer must flow at a sufficient rate (have low enough viscosity) to cover the entire crack before it is polymerized, or full healing capacity will not be reached. Finally, the catalyst must quickly dissolve into monomer in order to react efficiently and prevent the crack from spreading further.
Source: http://en.wikipedia.org/wiki/File:Reaction_for_wiki_page.png
INTRODUCTION
• Concrete is a material which is the most widely used building material in the world.
• Natural processes such as weathering, faults, land subsidence, earthquakes, and human activities creates cracks in concrete structures.
• Concrete expands and shrinks with changes in moisture and temperature and this tendency to shrink and expands causes cracks in concrete.
• We do not like cracks in concrete because cracks form an open pathway to the reinforcement and can lead to durability problems like corrosion of the steel bars.
• These cracks should be repaired because they can reduce the service life of structure.
• In case of historical monuments cracks spoils the appearance of structure.
• Remediation of already existing cracks has been subject of research for many years.
• The various product such as structural epoxy, resins, epoxy mortar, and other synthetic mixtures are used as filling material but they are not environmentally friendly not even safe for human health.
• Here are some four possible mechanisms given for self healing of concrete which are as under :
1.) Formation of material like calcite
2.) Blocking of the path by sedimentation of Particles
3.) Continued hydration of cement particles
4.) Swelling of the surrounding cement matrix.
Different healing mechanisms
The healing process is similar to the way human skin repairs itself: A paper cut heals much faster than an inch-wide gash. When Li’s concrete develops hairline cracks, the dry composite is exposed to the moisture in the air, which it absorbs. As it does, it “grows” new concrete, filling in the minuscule cracks. Meanwhile, calcium ions inside the cracked concrete mix with moisture and carbon dioxide from the air, creating a calcium carbonate material similar to what seashells are made of. This enables the concrete to regain its initial degree of strength.
“If self-healing concrete can minimize cracks, you should get a longer life and a more sustainable structure,” says Andrew Herrmann, a partner at New York City infrastructure transportation consulting firm Hardesty & Hanover.
In 2002 Li debuted a precursor concrete material that was bendable (but not self-healing). This bendable concrete has been used in the core of several high-rise buildings in Japan–including a residential tower in Osaka completed this year–to absorb energy during earthquakes.
Li figures he’s five years away from putting his self-healing concrete into bridges and roads. It needs to go through years of testing before it can be approved for use. A patent for the newfangled concrete was granted to the University of Michigan in August this year; Li and his former graduate student En-hua Yang were named the inventors. The university is currently in talks with building materials companies about licensing agreements.

The precursor to Li’s self-healing concrete: a bendable version.
When Li moved to the U.S. from his native Hong Kong in 1973, he wanted to invent a way to reduce construction noise. Growing up, he saw and heard the endless construction of an expanding Hong Kong. At Brown University he got undergraduate degrees in economics and mechanical engineering and, in 1981, a Ph.D. in solid and structural mechanics. While at Brown he became baffled by the fact that a country as wealthy as the U.S. had such run-down roads and bridges, especially compared with Hong Kong. That pushed him to find a better solution.
Li’s self-healing concrete would cost about three times more than the standard item, he says, but pay for itself in reduced repair work. He estimates that the Grove Street Bridge, which crosses highway I-94 in Ypsilanti, Mich. and was built with traditional concrete, will average $350,000 a year in maintenance, user and environmental costs–its so called “life-cycle cost”–over 60 years. The same bridge, if built with Li’s self-healing concrete, ought to have a 50% lower life-cycle cost. That would add up to a savings of $11 million, potentially justifying a much higher initial price tag.
Armed with $9 million from the National Institute of Standards & Technology and $10 million from various sponsors, including the Michigan Department of Transportation, Li is working on his next project: a concrete that can not only heal itself but also tell you when it has been damaged. A concrete that can talk back to monitoring systems–that could be the ticket to getting an “A” grade on U.S. infrastructure.
A handful of drizzly days would be enough to mend a damaged bridge made of a new self-healing concrete developed by researches at the University of Michigan. Self-healing is possible because the material is designed to bend and crack in narrow hairlines rather than break and split in wide gaps like traditional concrete.
“It’s like if you get a small cut on your hand, your body can heal itself,” said Victor Li, a professor of materials science and engineering at the university. “But if you have a large wound, your body needs help. You might need stitches. We’ve created a material with such tiny crack widths that it takes care of the healing by itself.”
Li and his team have been working for the past 15 years on a bendable, engineered cement composite, or ECC. In his new recipe, extra-dry cement in the concrete on the crack surfaces can react with water and carbon dioxide to heal and form a thin white scar of calcium carbonate—a strong compound found naturally in seashells.
By reversing the typical deterioration process, the concrete could reduce the cost and environmental impacts of making new structures, Li said. And repairs would last longer. The American Society of Civil Engineers recently gave the country’s roads, bridges, water systems and other infrastructure a “D” grade for health. The federal stimulus package includes more than $100 billion for public works projects.
In the lab, self-healed specimens recovered most if not all of their original strength after researchers subjected them to a 3 percent strain—enough to severely deform metal or catastrophically fracture traditional concrete. Traditional concrete fractures and can’t carry a load at .01 percent strain.
“When we load it again after it heals, it behaves just like new, with practically the same stiffness and strength,” Li said.
Traditional concrete is considered a ceramic. Brittle and rigid, it can suffer catastrophic failure when strained in an earthquake or by routine overuse, Li said. More flexible than traditional concrete, ECC acts more like metal than glass. Studded with specially-coated reinforcing fibers that hold it together, ECC bends without breaking and remains intact and safe to use at tensile strains up to 5 percent.
Today, builders reinforce concrete structures with steel bars to keep cracks as small as possible. But they’re not small enough to heal, so water and de-icing salts can penetrate to the steel, causing corrosion that further weakens the structure. Li’s self-healing concrete needs no steel reinforcement to keep crack width tight, which eliminates corrosion.
“Our hope is that when we rebuild our roads and bridges, we do it right, so that this transportation infrastructure does not have to undergo the expensive repair and rebuilding process again in another 5 to 10 years,” Li said. “Also, rebuilding with self-healing, bendable concrete would allow a more-harmonious relationship between the built and natural environments by reducing the energy and carbon footprints of these infrastructures.”
The University of Michigan is pursuing a patent on the ECC formula and is seeking commercialization partners to help bring the technology to market.
A paper about the material is published online in Cement and Concrete Research. The research is funded by the National Science Foundation and a China National Scholarship.
Definition of Internal Curing (IC)
The ACI-308 Code states that “internal curing refers to the process by which the hydration of cement occurs because of the availability of additional internal water that is not part of the mixing Water.” Conventionally, curing concrete means creating conditions such that water is not lost from the surface i.e., curing is taken to happen ‘from the outside to inside’. In contrast, ‘internal curing’ is allowing for curing ‘from the inside to outside’ through the internal reservoirs (in the form of saturated lightweight fine aggregates, superabsorbent polymers, or saturated wood fibers) Created. ‘Internal curing’ is often also referred as ‘Self–curing.’
Need for Self–curing
When the mineral admixtures react completely in a blended cement system, their demand for curing water (external or internal) can be much greater than that in a conventional ordinary Portland cement concrete. When this water is not readily available, due to depercolation of the capillary porosity, for example, significant autogenous deformation and (early-age) cracking may result.
Due to the chemical shrinkage occurring during cement hydration, empty pores are created within the cement paste, leading to a reduction in its internal relative humidity and also to shrinkage which may cause early-age cracking. This situation is intensified in HPC (compared to conventional concrete) due to its generally higher cement content, reduced water/cement (w/ c) ratio and the pozzolanic mineral admixtures (fly ash, silica fume). The empty pores created during self-desiccation induce shrinkage stresses and also influence the kinetics of cement hydration process, limiting the final degree of hydration. The strength achieved by IC could be more than that possible under saturated curing conditions.
Water Required for Self–curing
It depends upon chemical and autogenous shrinkages expected during hydration reactions.
Types of Shrinkage Drying
Shrinkages may occur at earlyages or at later ages over a longer period; different types of shrinkages may be identified as :
Drying shrinkage, autogenous shrinkage, thermal shrinkage, and carbonation shrinkage.
Products being less than that of the reactants (cement and water). For example: Hydration of tricalcium silicate:
C3S + 5.3 H -> C1.7SH4 + 1.3 CH
Bacterial concrete
• The “Bacterial Concrete” is a concrete which can be made by adding bacteria in the concrete that are able to constantly precipitate calcite, this phenomenon is called microbiologically induced calcite precipitation.
• It is process by which, living organisms form an inorganic solids.
• It is same process as we people are producing teeth and bones.
Autogenous Shrinkage
It is as a volume change in concrete occurring without moisture transfer from the environment intoconcrete. It is due to the internal chemical and structural reactions of the concrete. Autogenous shrinkage is prominent in HPCs due to the reduced amount of water and increased amount of various binders used.
At early ages (the first few hours), before the concrete has formed a hardened skeleton, autogenous shrinkage is often due to only chemical shrinkage. At later ages (>1+days), the autogenous shrinkage can also result from self-desiccation since the hardened skeleton resists the chemical shrinkage.
The external (macroscopic) dimensional reduction of the cementitious system under isothermal sealed curing conditions; can be 100 to 1000 micro strains.
Self-desiccation
It is the localized drying resulting from a decreasing relative humidity (RH) which could be the result of the cement requiring extra water for hydration. It is the reduction in the internal relative humidity of a sealed system when empty pores are generated.
Potential of Selfdesiccation Prominent in HPC/ HSC
The finer porosity of HSC/HPC (with a low w/c), causes the water meniscus to have a greater radius of curvature, causing large compressive stress on the pore walls, leading to greater autogenous shrinkage as the paste is pulled inwards. Self–desiccation is only a risk when there is not enough localized water in the paste for the cement to hydrate and it occurs the water is drawn out of the capillary pore spaces between the solid particles. At later ages, a strong correlation exists between internal relative humidity and free autogenous shrinkage.
Mineral admixtures, such as fly ash and silica fume, in concrete tend to refine the pore structure towards a finer microstructure thereby water consumption will be increased and the autogenous shrinkage due to self-desiccation will be increased.
Inter-dependance of Autogenous& Chemical Shrinkages
Chemical shrinkage creates empty pores within hydrating paste and stress generated is stimated by equation:
The above equation is only approximate for a partiallysaturatedvisco-elastic material such as hydrating cement paste, but still provides insight into the physical mechanism of autogenous shrinkage and the importance of various physical parameters The internal drying is analogous to external drying shrinkage.
Early External Water Curing and Cracks in HPC
Reduction of autogenous shrinkage due to external curing in HPCs is possible for first one or two days when the capillary pores are yet interconnected. Early water curing can lead to higher strain gradients when the skin of the concrete becomes well cured (no shrinkage) whereas, autogenous shrinkage, which is generally difficult to control, begins at the interior of the concrete. These problems can be mitigated by use of a pre-soaked LWA.
Monitoring of Self – curing
This can be done by:
i. Measuring weight-loss
ii. X-Ray powder diffraction
iii. X-Ray microchromatography
iv. Thermogravimetry (TGA) measurements
v. Initial surface absorption tests (ISAT)
vi. Compressive strength
vii. Scanning electron microscope (SEM)
viii. Change internal RH with time
ix. Water permeability
x. NMR spectroscopy
Advantages of Internal Curing
a. Internal curing (IC) is a method to provide the water to hydrate all the cement, accomplishing what the mixing water alone cannot do. In low w/c ratio mixes (under 0.43 and increasingly those below 0.40) absorptive lightweight aggregate, replacing some of the sand, provides water that is desorbed into the mortar fraction (paste) to be used as additional curing water. The cement, not hydrated by low amount of mixing water, will have more water available to it.
b. IC provides water to keep the relative humidity (RH) high, keeping self-desiccation from occurring.
c. IC eliminates largely autogenous shrinkage.
d. IC maintains the strengths of mortar/concrete at the early age (12 to 72 hrs.) above the level where internally & externally induced strains can cause cracking.
e. IC can make up for some of the deficiencies of external curing, both human related (critical period when curing is required is the first 12 to 72 hours) and hydration related (because hydration products clog the passageways needed for the fluid curing water to travel to the cement particles thirsting for water). Following factors establish the dynamics of water movement to the unhydrated cement particles:
i. Thirst for water by the hydrating cement particles is very intense,
ii. Capillary action of the pores in the concrete is very strong, and
iii. Water in the properly distributed particles of LWA (fine) is very fluid.
Concrete Deficiencies that IC can Address
The benefit from IC can be expected when
• Cracking of concrete provides passageways resulting in deterioration of reinforcing steel,
• low early-age strength is a problem,
• permeability or durability must be improved,
• rheology of concrete mixture, modulus of elasticity of the finished product or durability of high fly-ash concretes are considerations.
• Need for: reduced construction time, quicker turnaround time in precast plants, lower maintenance cost, greater performance and predictability.
Improvements to Concrete due to Internal Curing
• Reduces autogenous cracking,
• largely eliminates autogenous shrinkage,
• Reduces permeability,
• Protects reinforcing steel,
• Increases mortar strength,
• Increases early age strength sufficient to withstand strain,
• Provides greater durability,
• Higher early age (say 3 day) flexural strength
• Higher early age (say 3 day) compressive strength,
• Lower turnaround time,
• Improved rheology
• Greater utilization of cement,
• Lower maintenance,
• use of higher levels of fly ash,
• higher modulus of elasticity, or
• through mixture designs, lower modulus
• sharper edges,
• greater curing predictability,
• higher performance,
• improves contact zone,
• does not adversely affect finishability,
• does not adversely affect pumpability,
• reduces effect of insufficient external curing.
Dutch researchers have developed a self-healing cement that turns water-logged cracks to its advantage.
No product evokes a sense of solidity and sturdiness the way concrete does. However, the tiniest of cracks in an otherwise colossal slab will inevitably lead to structural degradation, leakages and costly repairs.
It is precisely this problem that two Dutch researchers from Delft Technical University have been working on. Beginning in 2006, HenkJonkers, a microbiologist, and Eric Schlangen, a specialist in concrete development, sought to develop a self-healing cement [pictured] that would stop cracks from forming in the concrete, thereby extending the life of constructions.
Microcracks have a width of just 0.2-0.4mm, but that’s enough for water to leak in, degrading the concrete and the steel reinforcements embedded within it. Using the potentially damaging water to their advantage, Jonkers and Schlangen added a healing agent into the concrete, composed of bacterial spores and a feed.
Jonkers explains that the incoming water activates the bacterial spores, causing them to convert the feed into limestone, which seals the crack. Tunnels, basements and highway infrastructure are ideal ‘wet environments’ which will benefit from this innovation.
Rachel Armstrong, senior lecturer in the School of Architecture and Construction at the University of Greenwich, calls the project “a landmark in developing ‘living’ materials”.
However, “the production of calcite does not appear to me to actually increase the structural integrity of the concrete: [it] just stops the progression of the faults”, Armstrong added.
While this bacteria-infused cement is not alone in the world of self-healing concrete, Jonkers and Schlangen’s concrete has succeeded in healing cracks 10 times longer than other methods.
At present, the biggest challenge is producing large-scale quantities of the healing agent at affordable costs.
With the hope of long-term savings from the increased life expectancy of constructions, several companies and stakeholders have expressed interest in the product, including the Dutch ministry of road affairs. The two researchers expect their concrete to enter the market in about four years.
Cracking of concrete is a common phenomenon. Without immediate and proper treatment, cracks in concrete structures tend to expand further and eventually require costly repairs. Even though it is possible to reduce the extent of cracking by available modern technology, remediation of cracks in concrete has been the subject of research for many years. There are a large number of products available commercially for repairing cracks in concrete: structures epoxy, resins, epoxy mortar and other synthetic mixtures. Cracks and fissures are a common problem in building structures, pavements, and historic monuments. We have introduced a novel technique in fixing cracks with environmentally friendly biological processes that is a continuous self-remediating process. In the study, Bacillus pasteurii that is abundant in soil has been used to induce CaC[O.sub.3] precipitation. It is therefore vital to understand the fundamentals of microbial participation in crack remediation.
Definition: The “Bacterial Concrete” is a concrete which can be made by embedding bacteria in the concrete that are able to constantly precipitate calcite. This phenomenon is called microbiologically induced calcite precipitation. It has been shown that under favorable conditions for instance Bacillus Pasteruii, a common soil bacterium, can continuously precipitate a new highly impermeable calcite layer over the surface of an already existing concrete layer. The favorable conditions do not directly exist in a concrete but have to be created.
Chemistry of the Process
Microbiologically enhanced crack remediation (MECR) utilizes a biological byproduct, CaC[O.sub.3] which has shown a wide range of application potential as a sealant. Its prospective applications include remediation of surface cracks and fissures in various structural formations, in-base and sub-base stabilization, and surface soil consolidation. In principle, MECR continues as microbial metabolic activities go on. This inorganic sealant not only is environmentally innocuous but also persists in environments for a prolonged period.
Microbiologically induced calcium carbonate precipitation (MICCP) is comprised of a series of complex biochemical reactions, including concomitant participations of Bacillus pasteurii, urease (urea amidohydrolase), and high pH. In this process, an alkalophilic soil microorganism, Bacillus pasteurii, plays a key role by producing urease that hydrolyzes urea to ammonia and carbon dioxide. The ammonia increases the pH in surroundings, which in turn induces precipitation of CaC[O.sub.3], mainly as a form of calcite. In aqueous environments, the overall chemical equilibrium reaction of calcite precipitation can be described as:
[Ca.sup.2+] C[O.sub.3.sup.2-] [right arrow] CaC[O.sub.3] [down arrow] (1)
Possible biochemical reactions in Urea-Ca[Cl.sub.2] medium to precipitate CaC[O.sub.3] at the cell surface can be summarized as follows:
[Ca.sup.2+] + Cell [right arrow] Cell-[Ca.sup.2+] (2)
C1 + HC[O.sub.3] + N[H.sub.3] [right arrow] N[H.sub.4]Cl + C[O.sub.3.sup.2-] (3)
Cell-[Ca.sup.2+] + C[O.sub.3.sup.2-] [right arrow] Cell-CaC[O.sub.3] [down arrow] (4)
Immobilization of Bacteria
* It is the technique in which microorganisms encapsulated in different porous material to maintain high metabolic activities and protect from adverse environment.
* For immobilization different materials like polyurethane (PU) polymer, lime, silica, fly ash can be used.
* PU can be used widely because of its mechanically strong and biochemically inert characteristics.
* PU mix open cell foam as a result of condensation of polycyanates (R-CNO) and polyols (R-OH).
Evidence of Calcite Precipitation Induced Bacillus Pasteurii
Upon polymerization, PU foam is pliable and elastic with open-cell structure of matrices (Figure. 1.a). Micrographs (Figures. 1.b-1.c) showing cell-laden PU matrices indicate that immobilization caused no apparent morphological damage to the cells and microorganisms are entrapped throughout the polymer matrices where cells are adhered or embedded with some clumping. As shown in Figs. 1.d and 1.e, calcite precipitation occurred throughout the entire matrices, including the inside of pores as well as the surface areas. It is also apparent that calcite crystals grow around the microorganisms and PU matrices (Figure. 1.f).
Strength and durability performance of bacterial concrete:
* The performance of MICCP in concrete remediation was examined using hairline-cracked cement mortar beams remediated in the medium with B. pasteurii. Various levels of performance enhancement was observed in the treated specimens;
* Reduction of the mean expansion due to the alkali aggregate reactivity by 20%.
* Reduction of sulfate effects by 38%; reduction of the mean expansion by 45% after freeze-thaw cycle; and higher retaining rates (30% more) of the original weight.
* The microbiological enhancement of concrete was further supported by SEM analysis evidencing that a new layer of calcite deposit provided an impermeable sealing layer, increasing the durability of concrete against the freeze-thaw cycles and chemicals with extreme pH.
Microbiological precipitation of caco3: Effect of ammonia and pH on growth of cell:
* Calcium carbonate precipitation appeared to be correlated with the growth of B. pasteurii and was completed within 16 hr following inoculation. A considerable amount of ammonia was produced even during the stationary phase of cell growth.
* The pH of the medium also increased slowly as ammonia production increased, but did not directly increase with the growth of cells.
Efficiency of bacteria as biosealent:
Efficiency of filling material for crack remediation.
The results suggest that PU provides cells with protection from a high pH of concrete and further supports the growth of bacteria more efficiently than other filling materials. (Increase in Compressive Strength due to MECR).
Experimental Procedures
Micro-Organisms and Growth Condition
A stock culture of B.pasteurii is generally maintained in a solid medium containing : 10g trypcase; 5g yeast extract; 4.5g tricine; 5g [(N[H.sub.4]).sub.2]S[O.sub.4]; 2g glutamic acid and final concentration of 1.6% agar, which is autoclaved separately and added after-wards. For quantity culture use in MICCP B. Pasteurii is cultured in yeast extract (YE) broth medium with 30 [degrees]C for 24-30h with aeration facilities.
Transfer of bacteria
After sufficient growth of bacteria in laboratory same is transfer to cracked mortar cubes by mixing with sand and required amount of cell concentration of bacteria.
Conclusions
Based on the experimental program carried out at SV National Institute of Technology, Surat (INDIA) conclusions are drawn as follows:
The positive potential of MICCP that has been demonstrated in our study offer an interesting concept of the crack remediation technique in various Structures. The following summarizes our preliminary findings on MICCP.
Rod shape impression which consists with dimension of B. Pasteurii on the calcite crystal further confirm that bacteria serves as nucleation site for calcite crystals and also creates an alkaline environment surrounding to them includes more precipitation of calcite.
A concentration of 9.0x[10.sup.8] cells per ml is most suitable for the maximum compressive strength. Specimens with higher concentration do not give higher compressive strength values probably because the greater population of bacteria does not have enough nutrients to multiply.
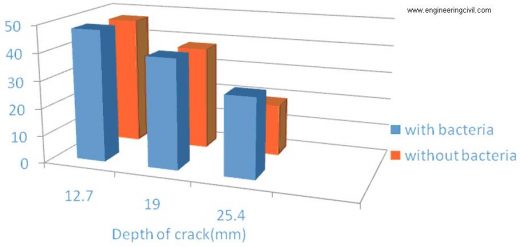
Comparisons of Compressive Strength of Specimens having Various Crack depths with single immobilize cell concentration.
B. Pasteurii however, has an ability to produce the endospore, a dormant form of the cell, to endure extreme environment so if in future cracks treated with this technique widens then again B.pasteurii starts metabolic activity, which leading to accumulation of insoluble CaC[O.sub.3].
The optimum concentration of B.pasteurii should be decided with considering parameters like crack size, frequency of reaction mix application, length of microbial treatment, remediation temperature and material for immobilization, environmental condition etc.
Based on the observation made in this study, it is clear that MICCP has excellent potential in cementing concrete as well as several other types of structural and nonstructural cracks.
Source:http://bouncingideas.files.wordpress.com/2012/02/microencapsulated-materials-self-healing-materials.png

Source:http://autonomic.beckman.illinois.edu/rupture.html
Polymer breakdown
From a molecular perspective, traditional polymers yield to mechanical stress though cleavage of sigma bonds. While newer polymers can yield in other ways, traditional polymers typically yield through homolytic or heterolytic bond cleavage. The factors that determine how a polymer will yield include: type of stress, chemical properties inherent to the polymer, level and type of solvation, and temperature.
From a macromolecular perspective, stress induced damage at the molecular level leads to larger scale damage called microcracks. A microcrack is formed where neighboring polymer chains have been damaged in close proximity, ultimately leading to the weakening of the fiber as a whole.
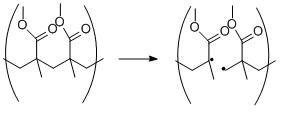
Scheme 1.Homolytic cleavage of poly(methyl methacrylate) (PMMA).
Polymers have been observed to undergo homolytic bond cleavage through the use of radical reporters such as DPPH (2,2-diphenyl-1-picrylhydrazyl) and PMNB (pentamethylnitrosobenzene.) When a bond is cleaved homolytically, two radical species are formed which can recombine to repair damage or can initiate other homolytic cleavages which can in turn lead to more damage.
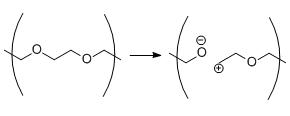
Scheme 2.Heterolytic cleavage of polyethylene glycol.
Polymers have also been observed to undergo heterolytic bond cleavage through isotope labeling experiments. When a bond is cleaved heterolytically, cationic and anionic species are formed which can in turn recombine to repair damage, can be quenched by solvent, or can react destructively with nearby polymers.
Reversible bond cleavage
Certain polymers yield to mechanical stress in an atypical, reversible manner.Diels-Alder-based polymers undergo a reversible cycloaddition, where mechanical stress cleaves two sigma bonds in a retro Diels-Alder reaction. This stress results in additional pi-bonded electrons as opposed to radical or charged moieties.
Supramolecular breakdown
Supramolecularpolymers are composed of monomers that interact non-covalently. Common interactions include hydrogen bonds, metal coordination, and van der Waals forces. Mechanical stress in supramolecular polymers causes the disruption of these specific non-covalent interactions, leading to monomer separation and polymer breakdown.
Reversible healing polymers
Reversible systems are polymeric systems that can revert to the initial state whether it is monomeric, oligomeric, or non-cross-linked. Since the polymer is stable under normal condition, the reversible process usually requires an external stimulus for it to occur. For a reversible healing polymer, if the material is damaged by means such as heating and reverted to its constituents, it can be repaired or “healed” to its polymer form by applying the original condition used to polymerize it.
Covalently bonded system
Diels-Alder and retro-Diels-Alder
Among the examples of reversible healing polymers, the Diels-Alder (DA) reaction and its retro-Diels-Alder (RDA) analogue seems to be very promising due to its thermal reversibility. In general, the monomer containing the functional groups such as furan or maleimide form two carbon-carbon bonds in a specific manner and construct the polymer through DA reaction. This polymer, upon heating, breaks down to its original monomeric units via RDA reaction and then reforms the polymer upon cooling or through any other conditions that were initially used to make the polymer. During the last few decades, two types of reversible polymers have been studied: (i) polymers where the pendant groups, such as furan or maleimide groups, cross-link through successive DA coupling reactions; (ii) polymers where the multifunctional monomers link to each other through successive DA coupling reactions.
Cross-linked polymers
In this type of polymer, the polymer forms through the cross linking of the pendant groups from the linear thermoplastics. For example, Saegusaet al. have shown the reversible cross-linking of modified poly(N-acetylethyleneimine)s containing either maleimide or furancarbonyl pendant moideties. The reaction is shown in Scheme 3. They mixed the two complementary polymers to make a highly cross-linked material through DA reaction of furan and maleimide units at room temperature, as the cross-linkedpolymer is more thermodynamically stable than the individual starting materials. However, upon heating the polymer to 80 °C for two hours in a polarsolvent, two monomers were regenerated via RDA reaction, indicating the breaking of polymers. This was possible because the heating energy provided enough energy to go over the energy barrier and results in the two monomers. Cooling the two starting monomers, or damaged polymer, to room temperature for 7 days healed and reformed the polymer.
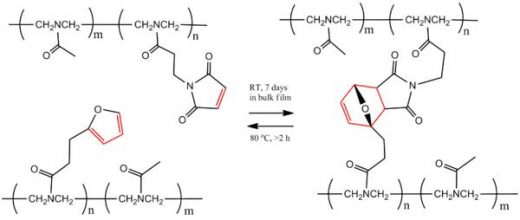
Scheme 3. Reversible polymer cross-linking via Diels-Aldercycloaddition reaction between furan and maleimide.
The reversible DA/RDA reaction is not limited to furan-meleimides based polymers as it is shown by the work of Schiraldiet al. They have shown the reversible cross-linking of polymers bearing pendent anthracene group with maleimides. However, the reversible reaction occurred only partially upon heating to 250 °C due to the competing decomposition reaction.
Polymerization of multifunctional monomers
In this type of polymer, the DA reaction takes place in the backbone itself to construct the polymer, not as a link. For polymerization and healing processes of a DA-step-growth furan-maleimide based polymer (3M4F) were demonstrated by subjecting it to heating/cooling cycles. Tris-maleimide (3M) and tetra-furan (4F) formed a polymer through DA reaction and, when heated to 120 °C, de-polymerized through RDA reaction, resulting in the starting materials. Subsequent heating to 90–120 °C and cooling to room temperature healed the polymer, partially restoring its mechanical properties through intervention. The reaction is shown in Scheme 4.
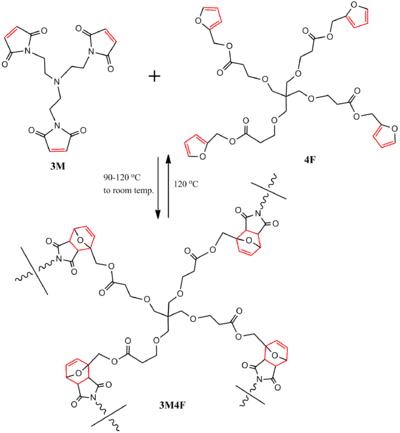
Scheme 4. Reversible highly cross-linked furan-maleimide based polymer network.
Thiol-based polymers
The thiol-based polymers have disulfide bonds that can be reversibly cross-linked through oxidation and reduction. Under reducing condition, the disulfide (SS) bridges in the polymer breaks and results in monomers, however, under oxidizing condition, the thiols (SH) of each monomer forms the disulfide bond, cross-linking the starting materials to form the polymer. Chujoet al. have shown the thiol-based reversible cross-linked polymer using poly(N-acetylethyleneimine). (Scheme 5)
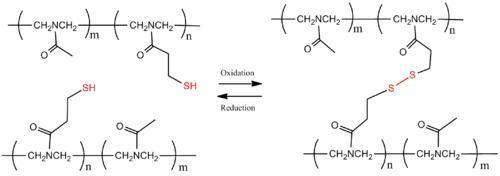
Scheme 5. Reversible polymer cross-linking by disulfide bridges.
Poly(urea-urethane)
A soft poly(urea-urethane) network leverages the metathesis reaction in aromatic disulphides to provide room-temperature self-healing properties, without the need for external catalysts. This chemical reaction is naturally able to create covalent bonds at room temperature, allowing the polymer to autonomously heal without an external source of energy.
Left to rest at room temperature, the material mended itself with 80 percent efficiency after only two hours and 97 percent after 24 hours.
Autonomic polymer healing
Self-healing polymers follow a three-step process very similar to that of a biological response. In the event of damage, the first response is triggering or actuation, which happens almost immediately after damage is sustained. The second response is transport of materials to the effected area, which also happens very quickly. The third response is the chemical repair process. This process differs depending on the type of healing mechanism that is in place. (e.g., polymerization, entanglement, reversible cross-linking). These self-healing materials can be classified in three different ways: capsule based, vascular, and intrinsic (which is listed as “Reversible healing polymers” above). While similar in some ways, these three ways differ in the ways that response is hidden or prevented until actual damage is sustained. Capsule based polymers sequester the healing agents in little capsules that only release the agents if they are ruptured. Vascular self-healing materials sequester the healing agent in capillary type hollow channels which can be interconnected one dimensionally, two dimensionally, or three dimensionally. After one of these capillaries is damaged, the network can be refilled by an outside source or another channel that was not damaged. Intrinsic self-healing materials, as you can see from above, do not have a sequestered healing agent but instead have a latent self-healing functionality that is triggered by damage or by an outside stimulus.
Thus far, all of the examples on this page require an external stimulus to initiate polymer healing (such as heat or light). Energy is introduced into the system to allow repolymerization to take place. This is not possible for all materials. Thermosetting polymers, for example, are not remoldable. Once they are polymerized (cured), decomposition occurs before the melt temperature is reached. Thus, adding heat to initiate healing in the polymer is not possible. Additionally, thermosetting polymers cannot be recycled, so it is even more important to extend the lifetime of materials of this nature.
Hollow tube approach
For the first method, fragile glass capillaries or fibers are imbedded within a composite material. (Note: this is already a commonly used practice for strengthening materials. See Fiber-reinforced plastic.) The resulting porous network is filled with monomer. When damage occurs in the material from regular use, the tubes also crack and the monomer is released into the cracks. Other tubes containing a hardening agent also crack and mix with the monomer, causing the crack to be healed. There are many things to take into account when introducing hollow tubes into a crystalline structure. First to consider is that the created channels may compromise the load bearing ability of the material due to the removal of load bearing material. Also, the channel diameter, degree of branching, location of branch points, and channel orientation are some of the main things to consider when building up microchannels within a material. Materials that don’t need to withstand much mechanical strain, but want self-healing properties, can introduce more microchannels than materials that are meant to be load bearing. There are two types of hollow tubes: discrete channels, and interconnected channels.
Discrete channels
Discrete channels can be built independently of building the material and are placed in an array throughout the material. When creating these microchannels, one major factor to take into account is that the closer the tubes are together, the lower the strength will be, but the more efficient the recovery will be. A sandwich structure is a type of discrete channels that consists of tubes in the center of the material, and heals outwards from the middle. The stiffness of sandwich structures is high, making it an attractive option for pressurized chambers. For the most part in sandwich structures, the strength of the material is maintained as compared to vascular networks. Also, material shows almost full recovery from damage.
Interconnected networks
Interconnected networks are more efficient than discrete channels, but are harder and more expensive to create. The most basic way to create these channels is to apply basic machining principles to create micro scale channel grooves. These techniques yield channels from 600–700 micrometers. This technique works great on the two-dimensional plane, but when trying to create a three-dimensional network, they are limited.
Direct Ink Writing
The Direct Ink Writing (DIW) technique is a controlled extrusion of viscoelastic inks to create three-dimensional interconnected networks. It works by first setting organic ink in a defined pattern. Then the structure is infiltrated with a material like an epoxy. This epoxy is then solidified, and the ink can be sucked out with a modest vacuum, creating the hollow tubes.
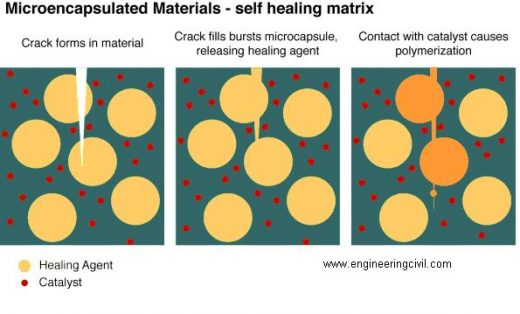
Microcapsule healing
This method is similar in design to the hollow tube approach. Monomer is encapsulated and embedded within the thermosetting polymer. When the crack reaches the microcapsule, the capsule breaks and the monomer bleeds into the crack, where it can polymerize and mend the crack

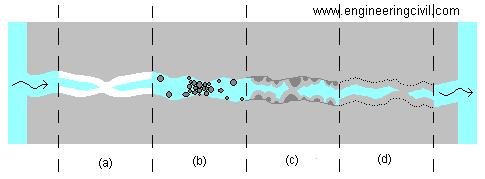
Figure 1. Depiction of crack propagation through microcapsule-imbedded material. Monomer microcapsules are represented by pink circles and catalyst is shown by purple dots.
A good way to enable multiple healing events is to use living (or unterminated chain-ends) polymerization catalysts. If the walls of the capsule are created too thick, they may not fracture when the crack approaches, but if they are too thin, they may rupture prematurely.
In order for this process to happen at room temperature, and for the reactants to remain in a monomeric state within the capsule, a catalyst is also imbedded into the thermoset. The catalyst lowers the energy barrier of the reaction and allows the monomer to polymerize without the addition of heat. The capsules (often made of wax) around the monomer and the catalyst are important maintain separation until the crack facilitates the reaction.
There are many challenges in designing this type of material. First, the reactivity of the catalyst must be maintained even after it is enclosed in wax. Additionally, the monomer must flow at a sufficient rate (have low enough viscosity) to cover the entire crack before it is polymerized, or full healing capacity will not be reached. Finally, the catalyst must quickly dissolve into monomer in order to react efficiently and prevent the crack from spreading further.
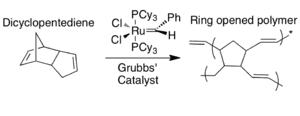
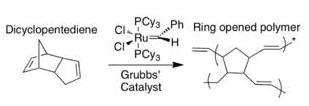
Scheme 6. ROMP of DCPD via Grubbs’ catalyst
This process has been demonstrated with dicyclopentadiene (DCPD) and Grubbs’ catalyst (benzylidene-bis(tricyclohexylphosphine)dichlororuthenium). Both DCPD and Grubbs’ catalyst are imbedded in an epoxyresin. The monomer on its own is relatively unreactive and polymerization does not take place. When a microcrack reaches both the capsule containing DCPD and the catalyst, the monomer is released from the core–shell microcapsule and comes in contact with exposed catalyst, upon which the monomer undergoes ring opening metathesis polymerization (ROMP). The metathesis reaction of the monomer involves the severance of the two double bonds in favor of new bonds. The presence of the catalyst allows for the energy barrier (energy of activation) to be lowered, and the polymerization reaction can proceed at room temperature. The resulting polymer allows the epoxycomposite material to regain 67% of its former strength.
Grubbs’ catalyst is a good choice for this type of system because it is insensitive to air and water, thus robust enough to maintain reactivity within the material. Using a live catalyst is important to promote multiple healing actions. The major drawback is the cost. It was shown that using more of the catalyst corresponded directly to higher degree of healing. Ruthenium is quite costly, which makes it impractical for commercial applications.
Carbon nanotube networks
Through dissolving a linear polymer inside a solid three-dimensional epoxy matrix, so that they are miscible to each other, the linear polymer becomes mobile at a certain temperature When carbon nanotubes are also incorporated into epoxy material, and a direct current is run through the tubes, a significant shift in sensing curve indicates permanent damage to the polymer, thus ‘sensing’ a crack. When the carbon nanotubes sense a crack within the structure, they can be used as thermal transports to heat up the matrix so the linear polymers can diffuse to fill the cracks in the epoxy matrix. Thus healing the material.
SLIPS
A different approach was suggested by Prof. J. Aizenberg from Harvard University, who suggested to use Slippery Liquid-Infused Porous Surfaces (SLIPS), a porous material inspired by the carnivorous pitcher plant and filled with a lubricating liquid immiscible with both water and oil. SLIPS possess self-healing and self-lubricating properties as well as icephobicity and were successfully used for many purposes. Self-healing in polymers and fibre-reinforced polymer composites
Catalytic liquid-based healing agents
Completely autonomous synthetic self-healing material was reported in 2001 on example of an epoxy system containing microcapsules. These microcapsules were filled with a (liquid) monomer. If a microcrack occurs in this system, the microcapsule will rupture and the monomer will fill the crack. Subsequently it will polymerise, initiated by catalyst particles (Grubbs catalyst) that are also dispersed through the system. This model system of a self healing particle proved to work very well in pure polymers and polymer coatings.
A hollow glass fibre approach may be more appropriate for self-healing impact damage in fibre-reinforced polymer composite materials. Impact damage can cause a significant reduction in compressive strength with little damage obvious to the naked eye. Hollow glass fibres containing liquid healing agents (some fibres carrying a liquid epoxy monomer and some the corresponding liquid hardener) are embedded within a composite laminate. Studies have shown significant potential.
Thermal solid-state healing agents
“Intrinsically” self-healing materials such as supramolecular polymers are formed by reversibly connected non-covalent bonds (i.e. hydrogen bond), which will disassociate at elevated temperatures. Healing of these supramolecullary based materials is accomplished by heating them and allowing the non-covalent bonds to break. Upon cooling, new bonds form and the material heals any damage. An advantage of this method is that no reactive chemicals or (toxic) catalysts are needed. However, these materials are not “autonomic” as they require the intervention of an outside agent to initiate a healing response.
Non-catalytic, non-thermal agents
A poly(urea–urethane) elastomeric networkcan spontaneously achieve healing in the absence of a catalyst. It is the metathesis reaction of aromatic disulphided (which naturally exchange at room temperature) that causes regeneration. It displayed 97% healing efficiency in just two hours and does not break when stretched manually. The tested poly(urea–urethane) composite is relatively soft.
Biomimetics
Self-healing materials are widely encountered in natural systems, and inspiration can be drawn from these systems for design. There is evidence in the academic literature of these biomimetic design approaches being used in the development of self-healing systems for polymer composites. In biology, for the minimum power to pump fluid through vessels Murray’s law applies. Deviation from Murray’s law is small however, increasing the diameter 10% only leads to an additional power requirement of 3%–5%. Murray’s law is followed in some mechanical vessels, and using Murray’s law can reduce the hydraulic resistance throughout the vessels. The DIW structure from above can be used to essentially mimic the structure of skin. Tooheyet al. did this with an epoxy substrate containing a grid of microchannels containing dicyclopentadiene (DCPD), and incorporated Grubbs’ catalyst to the surface. This showed partial recovery of toughness after fracture, and could be repeated several times because of the ability to replenish the channels after use. The process is not repeatable forever, because the polymer in the crack plane from previous healings would build up over time.
Further applications
Self-healing epoxies can be incorporated on to metals in order to prevent corrosion. A substrate metal showed major degradation and rust formation after 72 hours of exposure. But after being coated with the self-healing epoxy, there was no visible damage under SEM after 72 hours of same exposure.
History
Self healing materials only emerged as a widely recognized field of study in the 21st century. The first international conference on self-healing materials was held in 2007. The field of self-healing materials is related to biomimetic materials (materials inspired by living nature) as well as to other novel materials and surfaces with the embedded capacity for self-organization, such as the self-lubricating and self-cleaning materials.
However, some of the simpler applications have been known for centuries, such as the self repair of cracks in concrete. Related processes in concrete have been studied microscopically since the 19th century. A form of self healing mortar was known even to the ancient Romans.
America’s bridges are falling down. Victor Li says he knows a better way to build them. The civil and environmental engineering professor at the University of Michigan has invented a new kind of concrete that hardly ever cracks and, if it does, can repair itself.
A typical American bridge is built to last 50 years (and then only if it gets frequent roadbed replacements). Today the average age of U.S. bridges is 42 years. One-quarter of all bridges were deemed either structurally deficient or functionally obsolete last year by the U.S. Department of Transportation. In a March report the American Society of Civil Engineers gave U.S. infrastructure a grade of “D,” reflecting delayed maintenance and chronic underfunding; it estimates that $2.2 trillion is needed over the next five years to bring that grade up to a “B.”
Last year the world swallowed up 3 billion tons of cement, the active ingredient in concrete, according to the Portland Cement Association. (Concrete’s three other ingredients: gravel, sand and water.) Concrete has marvelous virtues, including a low cost and a high compression strength. Its main fault is brittleness. It needs steel inside for tensile strength.
Conclusion :
• It was found that beams with micro crack remediated with bacterial concentration of 8.6× 108 cells/ml of water regained 80% of its original strength.
• Higher concentration reduced the regaining strength of concrete.
• It was found that specimen with bacteria improved its permeability and resistance to alkaline environment, sulfate attack and freeze- thaw action.
• Thus we can say that crack remediated with bacteria can improve the strength and durability of structure.
• This all observation were done in America this results we cannot directly considered valid for our country because of difference in temperature, humidity, type of concrete, control on various parameters such as type of concrete mix, etc.
• In India porosity and permeability of concrete should be studied because they are the main causes of distress in many structures.
• If this method once studied in Indian environment then it can be used in crack remediation in many structures having more importance and containing hazardous material.
• In India Nuclear Power Corporation has started working on the research of bacterial concrete for using it in nuclear power plant.
REFERENCES:
-www.wikipedia.com
-www.fadooengineers.com
-www.selfhealingmaterials.com
-www.bouncingideas.com
We at engineeringcivl.com are thankful to Er Shubham Sunil Malu for submitting this paper to us.
If you have a query, you can ask a question here.